A Precision Medicine Company
Working to identify the root cause of multiple neurologic, psychiatric, and behavioral disorders.
Working to identify the root cause of multiple neurologic, psychiatric, and behavioral disorders.
The Cunningham Panel is an aid to a physician’s diagnosis of an infection-triggered autoimmune neuropsychiatric disorder.
Revolutionizing the way we look at mental disorders
Leaders in autoimmunity testing for neuropsychiatric illnesses
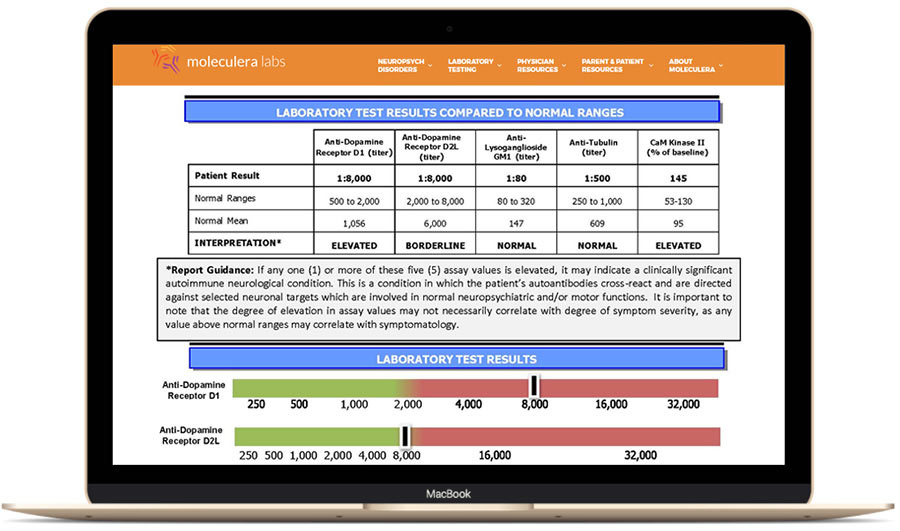
The Cunningham Panel™ helps clinicians identify whether a patient’s neurologic and/or psychiatric symptoms may be due to an infection-triggered basal ganglia encephalitis (BGE), which includes autoimmune neuropsychiatric syndromes such as PANS/PANDAS. Symptoms of BGE can mimic various mental illnesses.
The Cunningham Panel measures circulating levels of autoantibodies attacking brain receptors, as well as autoantibodies that stimulate the production of neurotransmitters in the basal ganglia. These interactions have the potential to disrupt neuronal functioning and can impact movement, behavior and cognition.
Check with your doctor as elevated levels of certain autoantibodies may indicate that neuropsychiatric symptoms could be due to BGE (a type of autoimmune encephalitis) rather than a primary psychiatric disorder.
Mental illness, behavioral disorder, or autoimmune encephalitis?
Common infections (i.e., strep, influenza, mycoplasma, Lyme disease, Babesia, Bartonella and coxsackie virus) can trigger autoimmune basal ganglia encephalitis. This condition can cause neurologic, behavioral and psychiatric symptoms which may include OCD, tics, anxiety, depression, and behaviors associated with autism spectrum disorders.
Science That Impacts Lives.
There is a growing body of scientific evidence documenting the connection between infection, inflammation, immune dysfunction and the development of neurologic and psychiatric illnesses. Following is a list of peer-reviewed journal articles by world-renowned researchers and clinicians investigating infection-induced autoimmune neurologic disorders.
Physicians worldwide are using the Cunningham Panel™ to help guide diagnosis and target treatments.
PIONEERING RESEARCH. LIFE-CHANGING TESTS
Remarkable results happen here.
Infection-triggered autoimmune disorders, such as PANS/PANDAS, which cause neuropsychiatric symptoms can be difficult to recognize. The following videos provide insight into the complexity of these disorders and demonstrate how the Cunningham Panel™ can assist physicians.
HEALTHCARE PROVIDERS: REQUEST A PERSONAL CONSULTATION


















